The Division of Hematology, Oncology, Transplant, and Cellular Therapy is committed to integrating research into the care of our patients. Our laboratories are located in the Wisconsin Institutes for Medical Research, which is connected to UW Health and the American Family Children’s Hospital.
The Division of Hematology, Oncology, Transplant, and Cellular Therapy is committed to integrating research into the care of our patients. Our laboratories are located in the Wisconsin Institutes for Medical Research, which is connected to UW Health and the American Family Children’s Hospital.
Clinical Research Programs
The major advances in treatment in childhood cancer have resulted from well-designed clinical trials. Thus the great majority of patients we care for participate in clinical trials. We offer access to cutting-edge treatments by writing our own institutional clinical trials based on the research lab data we have discovered, in addition to participating in multi-institutional group trials. Check out trials that are only available at UW Health. These include our activities in the:
- Children’s Oncology Group (COG)
- Pediatric Cancer Immunotherapy Trials Network (PedsCITN)
- Pediatric Transplantation & Cellular Therapy Consortium (PTCTC)
- Pediatric Neuro-oncology collaborative group
- Primary Immune Deficiency Treatment Consortium (PIDTC)
In our role as participants in the above clinical groups, members of our team have played leadership roles in the design, implementation and analyses of many national clinical research trials, particularly for the COG.
All faculty enroll patients with Hematologic Malignancies and Solid Tumors onto clinical trials offered through COG or exclusively at the UW. In addition, we have specific expertise in managing patients with neuroblastoma through cutting edge treatments such as anti-GD2 immunotherapy, MIBG therapy, or ex vivo expanded NK cells.
Clinical Trials
- Cancer Immunotherapy
- Targeted Radiotherapy
- Bone Marrow Transplant
- Hematologic Malignancies
- Solid Tumors
- Late Effects
- Neuro-oncology
Cancer Immunotherapy
For more information or questions, please contact us via email at PedsHemOncResearch@lists.wisc.edu or call us at 608-890-8070
Active (UW Only):
- TCR-αβ depleted haploidentical transplant – Carbone center information
- Immunocytokine + ex vivo expanded NK cells for neuroblastoma
- Treatment of cytomegalovirus (CMV) infections with viral-specific T cells
Active (multi-institutional):
- A phase II trial of tisagenlecleucel in first-line high-risk (HR) pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who are minimal residual disease (MRD) positive at the end of consolidation (EOC) therapy
- A pilot study evaluating the safety and efficacy of combining 1-131 MIBG therapy with an anti-GD2 monoclonal antibody and checkpoint blockade for children with relapsed or refractory neuroblastoma
- A phase 1/2 open label, basket study to assess the safety, tolerability and anti-tumor activity of Afamitresgene Autoleucel in pediatric subjects with MAGE-A4 positive tumors
In Development:
Targeted Radiotherapy
For more information or questions, please contact us via email at PedsHemOncResearch@lists.wisc.edu or call us at 608-890-8070
Active (UW Only):
Active (multi-institutional):
- Dose Escalation Study of CLR 131 in Children and Adolescents With Relapsed or Refractory Solid Tumors
- 67Cu-SARTATE™ peptide receptor radionuclide therapy administered to pediatric patients with high-risk, relapsed, refractory neuroblastoma
- Iobenguane I-131 or Crizotinib and standard therapy in treating younger patients with newly-diagnosed high-risk neuroblastoma or ganglioneuroblastoma
Bone Marrow Transplant
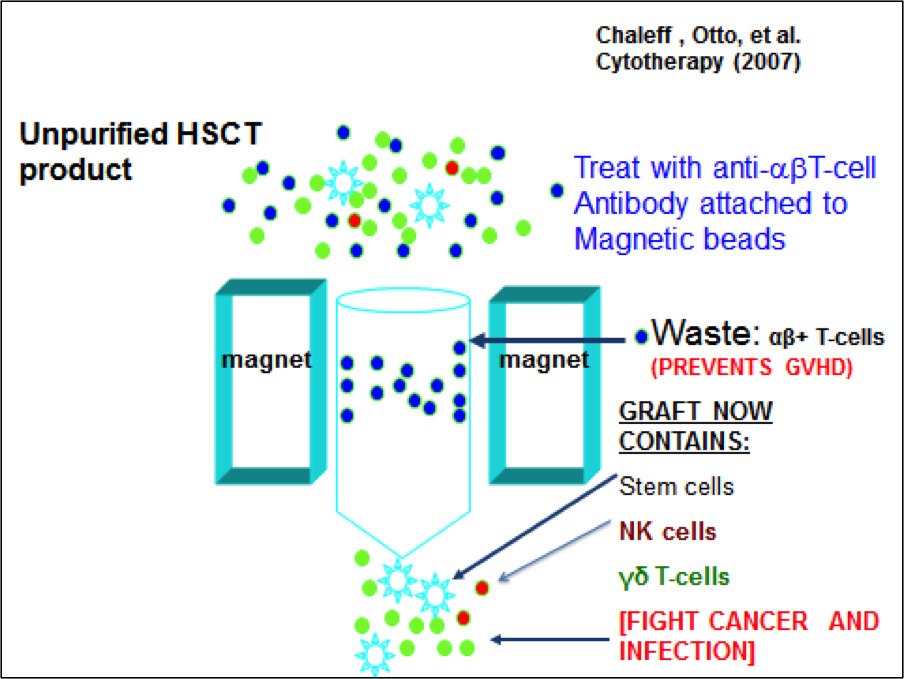
TCR-αβ+ and CD19+ Depleted KIR/KIR Ligand-mismatched Haploidentical Hematopoietic Stem Cell Transplant and Zoledronate for Pediatric Relapsed/Refractory Hematologic Malignancies and High Risk Solid Tumors
This phase I trial studies the safety of transplantation with a haploidentical donor (typically Mother or Father) peripheral blood stem cell graft depleted of TCRαβ+ cells (which cause GVHD) and CD19+ cells in conjunction with the immunomodulating drug, Zoledronate, given in the post-transplant period. Zoledronate expands gamma delta T cells, which have anti-tumor properties, speed up immune recovery but do not mediate GVHD.
For more information or questions, please contact us via email at PedsHemOncResearch@lists.wisc.edu or call us at 608-890-8070
Eligible Cancers
- Leukemia: Acute Myeloid & Acute Lymphoblastic
- Lymphoma: Hodgkin & Non-Hodgkin
- Myelodysplastic Syndrome
- Myeloproliferative Syndrome
- Sarcomas: Rhabdomyosarcoma, Ewing Sarcoma, Osteosarcoma
- Primitive Neuroectodermal Tumor
- Neuroblastoma
Ages eligible for study: 7 months to 21 years
Hematologic Malignancies
For more information or questions, please contact us via email at PedsHemOncResearch@lists.wisc.edu or call us at 608-890-8070
Eligible Leukemia and Lymphomas
- Acute Lymphoblastic Leukemia (ALL)
- Acute Myeloid Leukemia (AML)
- Chronic Myeloid Leukemia (CML)
- Juvenile Myelomonocytic Leukemia (JMML)
- Myelodysplastic Syndromes (MDS)
- Hodgkin’s Disease
- Non-Hodgkin Lymphomas (NHL)
Solid Tumors
For more information or questions, please contact us via email at PedsHemOncResearch@lists.wisc.edu or call us at 608-890-8070
Eligible Solid Tumors
| Sarcomas | Other Tumors | Other Oncologic Diseases |
|---|---|---|
Ewing SarcomaOsteosarcomaRhabdomyosarcoma |
NeuroblastomaWilms TumorHepatoblastomaRetinoblastomaGerm Cell TumorsEndocrine Tumors, including Adrenocortical carcinomaThyroid Tumors |
Langerhans’ Cell Histiocytosis (LCH)Hemophagocytic Lymphohistiocytosis (HLH)Vascular malformations (Hemangiomas and lymphangiomas)Neurofibromatosis (Malignant Peripheral Nerve Sheath Tumor) |
Late Effects
The Caring for Life Clinic is designed specifically to help the survivors of childhood cancer.
For more information or questions, please contact us via email at PedsHemOncResearch@lists.wisc.edu or call us at 608-890-8070
Clinic Goals
- Detect and treat problems related to being a childhood cancer survivor
- Share ongoing research findings with childhood cancer survivors
- Provide health maintenance education about potential risks and preventive behaviors
- Provide information to school personnel and employers to meet the special needs of survivors
- Provide emotional support to both survivors and family members
- Empower survivors of cancer to advocate for their rights
- Facilitate transition of medical care to an adult care system
Neuro-oncology
For more information or questions, please contact us via email at PedsHemOncResearch@lists.wisc.edu or call us at 608-890-8070
Eligible Brain & Spinal Tumors
- Medulloblastoma
- Astrocytoma
- Ependymoma
- Brain Stem Glioma
- Optic Glioma
- Atypical Teratoid/Rhabdoid tumor (ATRT)
- Craniopharyngioma