Anxiolytic Drugs
Drugs used to make children “more workable” and comfortable. Not sleepers. Particularly effective for noninvasive procedures or slightly painful procedures that do not require high immobilization and as adjuncts with analgesics for category 4.
Benzodiazepines (BNZs): Diazepam, Midazolam, Lorazepam
Pharmacodynamic Features
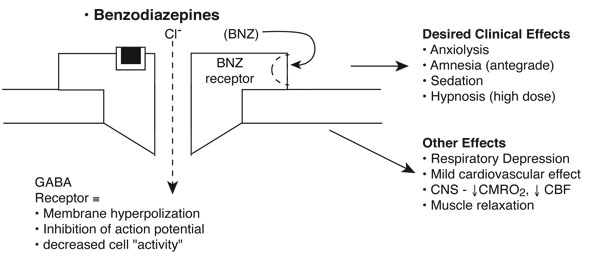
BNZs enhance gamma-aminobutyric acid (GABA) neurotransmission by binding to specific BNZ receptors on the GABAA receptor complex. They enhance chloride flux across ligand-gated chloride channels, resulting in membrane hyperpolarization and inhibition of the action potential.
Basics
The time of clinical onset and duration of action following single-dose administration is primarily related to lipid solubility.
| BNZs | Lipid Solubility* | Volume of Distribution | Clearance (mL/kg/min) | Elimination half-life (hours) | Metabolism | Active Metabolite |
|---|---|---|---|---|---|---|
| Diazepam | +++ | 0.7-1.7 | 0.2-0.5 | <20-40 >40 (critically ill) |
Oxidation ↓ Conjugation |
Desmethyldiazepam (t 1/2 β=80-70 hours) |
| Midazolam | +++ | 1.1-1.7 | 6.4-11.1 | 2-4 >6-10 (critically ill) |
Oxidation ↓ Conjugation |
α-Hydroxymidazolam (t 1/2 β=1 hour) |
| Lorazepam | + | 1.1-1.8 | 0.7-1.0 | 10-20 >40 (neonates) |
Conjugation | – |
*Lipid Solubility: (+) low, (++) medium, (+++) moderate, (++++) high
- Diazepam and midazolam have active metabolites and require both oxidation and conjugation.
- Lorazepam does not have metabolites and requires only conjugation.
- All BNZs are highly protein-bound.
| Benzodiazepine | Dose | Repeat Dose | Onset | Duration |
|---|---|---|---|---|
| Diazepam | 0.1-0.15 mg/kg | 0.05-0.1 mg/kg q 3-5 min |
<60 sec | 15-30 min |
| Midazolam | 0.05-0.1 mg/kg | 0.05 mg/kg q 3-5 min |
<60 sec | 15-30 min |
| Lorazepam | 0.05 mg/kg | .025-.05 mg/kg q 10-15 min |
2-3 min | 1-2 hrs |
| Administer | Dose | Clinical Onset | (+) | (-) |
|---|---|---|---|---|
| Intranasal | 0.2 mg/kg | 10-15 min | faster effects | irritating |
| Rectal | 0.3-0.4 mg/kg | 15-20 min. | dependable | not older children |
| Oral | 0.3-0.5 mg/kg | 20-30 min | easy admin | variable onset, bad taste |
Clinical Use (Most Experience with Midazolam)
Used in procedures associated with mild to moderate discomfort not requiring high levels of immobility (Pediatrics 1992;89:631). *Benzodiazepines are not reliable hypnotic agents (Eur J Anaesth 1991;8:29). Useful as adjuncts with analgesics. Antegrade amnesia is one of the most important clinical effects of BNZs, particularly in children undergoing invasive procedures.
Hypnotic Drugs (“Sleepers”)
Drugs used to make children sleep. Particularly effective for procedures requiring a high level of immobility.
Chloral Hydrate
Historically the most widely used hypnotic agent in children, has stood the “test of time.” Generally safe and effective.
Pharmacodynamic Features
Desired Clinical Effects
- Good sedation – hypnosis (similar to barbiturates)
Other Clinical Effects (Isolated Reports of Dealths in Dental Office)
- Respiratory: mild to moderate respiratory depressant effects
- Isolated oxygen desaturations (~5%)
- Bronchiolitis: oxygen desaturations (Pediatr Pulmonol 1988;5:96)
- Obstructive sleep apnea: upper airway obstruction (Pediatrics 1993;92:471)
- Cardiovascular: arrhythmias may occur following high doses or chronic use due to trichloroethanol (TCE) accumulation.
- Other: vomiting, agitation, tastes bad
Pharmacokinetic Features
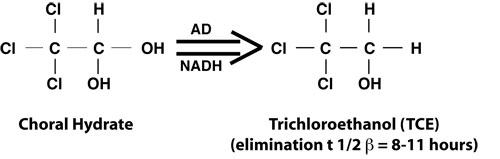
Chloral hydrate, 2,2,2-trichloroacetaldehyde, is metabolized by the liver by alcohol dehydrogenase (AD) to trichloroethanol, the major active metabolite.
Basics
- Dose:
- 50–75 mg/kg (oral, rectal)
- >75 mg/kg (high dose) (max. 2–2.5 g)
- Repeat dose: 20–25 mg/kg @ 20–25 min
- Induction time: ~15–25 min
- Recovery time: ~60–120 min
- Effectiveness: multifactorial
Clinical Use
Effective for nonpainful procedures requiring sedation or sleep (e.g., EEG, CT, MRI), particularly in children younger than 2 years. Success rates generally >85% for CT and MRI (AJR 1993;161:639, J Pediatr 1996;128:573).
Barbiturates: Potent Sedative, Hypnotic, and Anticonvulsant Drugs
Pharmacodynamic Features
Desired Clinical Effects
- Excellent sedation – hypnosis (dose-related)
Other Clinical Effects
- Respiratory: Despite direct respiratory depressant effects, generally tolerated well in healthy children. Infants most sensitive.
- Cardiovascular: Both negative inotropic and vasodilatory properties; few clinically significant effects in healthy individuals. Hemodynamic effects most pronounced with rapid administration and in hypovolemia.
- Neurologic: ↓ CBF, and ↓ CMRO2, anticonvulsant
- Other: excitatory phenomena such as laryngospasm, agitation<
Pharmacokinetic Features
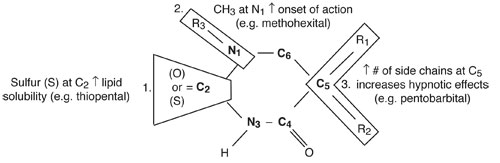
Substitutions at various positions of the barbituric acid ring confer different physical properties.
Pentobarbital: Most Frequently Used Barbiturate for Pediatric Sedation
Basics: Generally Safe and Effective
- Dose: 2–4 mg/kg (IV)
- Repeat dose: 1–2 mg/kg @ 5–10 min
- Induction time: 1–2 min
- Recovery time: 60 min
- Elimination t 1/2 b = 21–42 hrs
Clinical Use: Very Effective for Non-painful Procedures Requiring High Level of Immobility
(>95% success rate CT and MRI). Oxygen desaturations not uncommon (~5%) (Radiology 1986;161:105, AJNR 1988;9:955).
Analgesic Drugs
Drugs used for painful procedures. “Make sure that pain is treated promptly and adequately.” (Parent’s wish list for children with cancer, Durbin, 1997.)
Nonsteroidal Anti-Inflammatory Agents (Not a Sedative)
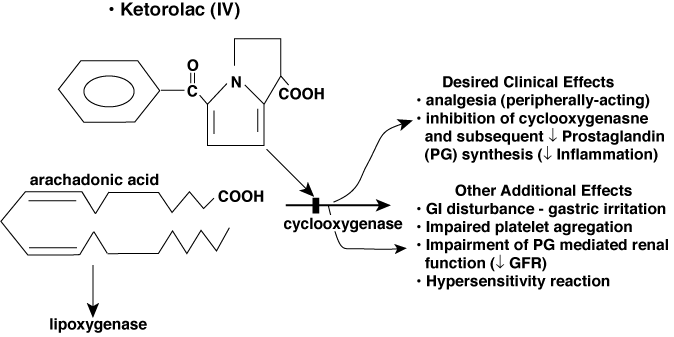
Use:
- Postoperative pain in children: Ketorolac 0.5/kg IV vs. MSO4
- Postoperative pain in critically ill children: Ketorolac 0.6/kg IV vs. MSO4 (Crit Care Med 1999;27:2786)
| Reaction to Treatment | Morphine (n=48) | Ketorolac (n=54) |
|---|---|---|
| Pain relief in first hour | 27 (56%) | 31 (57%) |
| Pain relief in first 2 hrs | 28 (58%) | 37 (68%) |
| Maximum pain relief in first 2 hrs | 21 (44%) | 27 (50%) |
| Required remedication within the first 4 hrs | 30 (63%) | 31 (58%) |
| Treatment failure (never achieved pain relief) | 7 (14%) | 2 (3.7%) |
Opioid Agonists
Morphine, Fentanyl, Meperidine, Methadone, Hydromorphone, Codeine, Alfentanil
Pharmacodynamic Features
Opioid agonists bind to specific opioid receptors (primarily µ receptors) distributed throughout the neuraxis. Opioids inhibit spontaneous neuronal firing and excitatory neurotransmitter release.
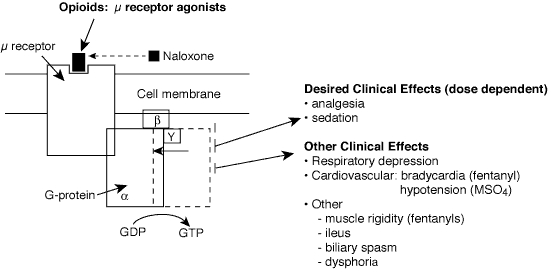
Basics
Clinical onset and duration distinguish morphine from fentanyl, despite similar half-lives.
| Opioid Agonist | Lipid Solubility* | Protein Binding (%) | Volume of distribution (L/kg) | Clearance (mL/kg/min) | Elimination half-life (hours) | Active Metabolites |
|---|---|---|---|---|---|---|
| Morphine | + | 30% (adults, children)
18-22% (neonates) |
3.2-4.7 | 12.4-15.2 | 2-4 (adults, children) 6-7 (infants) | Morphine-3-glucuronide (excitatory)
Morphine-6-glucuronide (active) |
| Fentanyl | ++++ | 80%-85% | 3.2-4.2 | 11.2-13.3 | 2.7 | – |
| Alfentanil | +++ | 92% | 0.86 | 6.4 | 1-1.6 | – |
| Methadone | +++ | 80%-85% | 7.1 | 5.4 | 15-30 | – |
*Lipid Solubility: (+) low, (++) medium, (+++) moderate, (++++) high
Notes:
- Morphine is much less protein-bound than fentanyl.
- Fentanyl does not have active metabolites.
- Morphine and fentanyl have similar elimination half-lives and much different lipid solubility.
| Opioid Agonist | Dose | Repeat Dose | Onset | Duration |
|---|---|---|---|---|
| Morphine | 0.05-0.2 mg/kg | 0.05 mg/kg q 10 min |
5-10 min | 3-4 hours |
| Fentanyl | *0.5-2 µg/kg | 0.5 mg/kg q 2-3 min |
2-3 min | 30-60 min |
*Administer over ~2 min
Clinical Use
Procedures associated with moderate to severe discomfort (pain), categories (3) and (4). BNZs augment opioid effects and provide amnesia.
Ketamine
Phencyclidine derivative with dissociative sedative, analgesic and amnestic properties.
Pharmacokinetic Features
Ketamine noncompetitively blocks the N-methyl-D aspartate (NMDA) receptor, part of a class of glutamate receptors mediating excitatory neurotransmission. Ion fluxes and subsequent excitatory neurotransmission are inhibited.
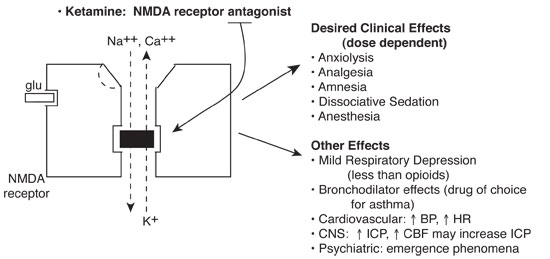
Basics
Clinically effective by a number of different routes. Administer with BNZ to attenuate neuropsychiatric effects and antisialagogue to reduce secretions. Ketamine is relatively contraindicated in any child with perceptual problems (visual, auditory, psychiatric). The single most severe adverse effect occurring in a healthy child is laryngospasm.
| NMDA Receptor Antagonist | Protein Binding (%) | Volume of distribution (L/kg) | Clearance (mL/kg/min) | Elimination half-life (hours) | Active Metabolites |
|---|---|---|---|---|---|
| Ketamine | 12% | 3 | 18 | 2-3 | Norketamine* |
*Predominates after enteral administration
| Administer | Dose | Repeat Dose | Clinical Onset | Clinical Peak | Clinical Duration |
|---|---|---|---|---|---|
| IV | 0.5-1 mg/kg* | 0.5 mg/kg (every 2-3 min) |
<60 sec | 1-2 min | 10-15 min |
| IM | 2-4 mg/kg | 2-4 mg/kg | 1-2 min | 2-4 min | 30-60 min |
| Oral | 6-10 mg/kg** | 6-10 mg/kg | ~10-15 min (variable) |
~20-45 min | 2-3 hours |
| Rectal | 6-8 mg/kg** | 6-8 mg/kg | ~5-10 min (variable) |
10-20 min | 2-3 hours |
*Midazolam 0.1 mg/kg (IV)
**Midazolam 0.2-0.3 mg/kg (p.o., p.v.)
Clinical Use
Procedures associated with moderate to severe discomfort and pain. Avoid in individuals with intracranial hypertension, systemic hypertension, or neuropsychiatric disorders and any child with visual or auditory problems (perceptual disorders) (Pediatrics 1992;90:537, Pediatrics 1997;99:427, Pediatrics 1998;102:956).