The goals of pediatric procedural sedation are to:
- maintain patient safety,
- provide effective pain control,
- reduce anxiety and psychological stress, and
- promote conditions conducive to successful performance of the procedure.
This is an accordion element with a series of buttons that open and close related content panels.
The Therapeutic Window
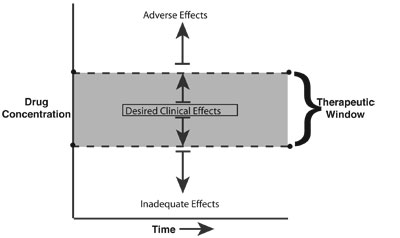
The therapeutic window refers to the drug concentration associated with the desired clinical effects. It describes the relationship between the drug concentration and its therapeutic and adverse effects.
- Drug concentrations above and below the “therapeutic window” result in inadequate and adverse clinical effects (e.g., hypoventilation), respectively.
- Large interpatient variability exists between drug concentration and clinical response.
- The goal is to administer the drug with the desired clinical effects, and the “right” drug (pharmacodynamics) at the “right” time (pharmacokinetics).
Pharmacokinetic and Pharmacodynamic Issues
A thorough understanding of the drug’s pharmacokinetic and pharmacodynamic profiles will optimize safe and effective patient sedation.
Pharmacodynamic issues deal with “what the drug does to the body” (including both desired and adverse clinical effects). These effects are similar within a given class of drugs (e.g., opioids) and are dependent on the target organ and sedative receptor system. Common drug-receptor systems include the following:
- Opioid agonist receptors: Fentanyl, morphine;
- Benzodiazepine receptors (gamma-aminobutyric acid, GABAA): midazolam, diazepam;
- NMDA receptors: ketamine;
- Central α2-receptors: clonidine.
Pharmacokinetic issues deal with “what the body does to the drug.” Pharmacokinetic properties distinguish drugs within a given class.
- Drug uptake by target organs is typically by passive diffusion and is driven by concentration gradients across cell membranes and tissue binding.
- Loading dose (LD). The appropriate loading dose of a sedative drug is determined by the desired plasma concentration (CP) associated with the desired clinical effect and the volume of distribution (VD) in which the drug is dispersed (LD = CP x VD).
- Physicochemical properties. Pharmacologic properties that are important in determining the speed and duration of action include the following:
- Blood flow (Vessel Rich Group, VRG) ≡ VRG organs get the drug first
- Protein binding ≡ only non-protein bound portion of drug crosses cellular membrane and is active.
- Degree of ionization (pKa) ≡ A– + H+⇔ AH, Unionized species cross the blood-brain barrier, Henderson Hassalbach equation (pH dependent).
- Lipid solubility ≡ the most important physicochemical property of sedative drugs determining onset of action and duration of effect. Highly lipid-soluble drugs penetrate and depart CNS quickly. Very lipid-soluble drugs typically have large VD.
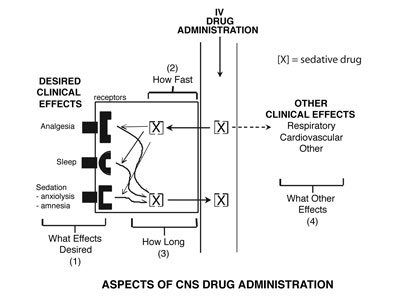
Sedation – Analgesia
Four questions should be asked when choosing a sedative drug for procedural sedation:
- What are the desired clinical effects?
- How quickly are the effects desired?
- What is the desired duration of effects?
- Are there any adverse “other” clinical effects?
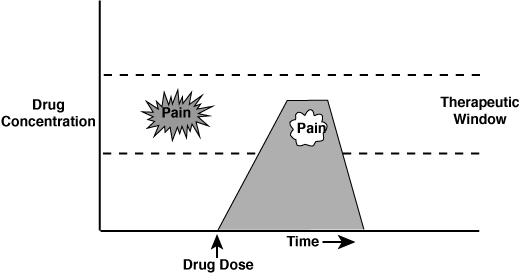
Optimizing Pharmacodynamics and Pharmacokinetics
“Give the “Right” Drug at the “Right” Time” approach makes use of the pharmacodynamic and pharmacokinetic properties of an individual drug. Optional use of the pharmacologic effects of a sedative drug is depicted in the figure below. In this example an analgesic agent is administered and achieves the desired concentration (clinical effect) during the painful procedure.
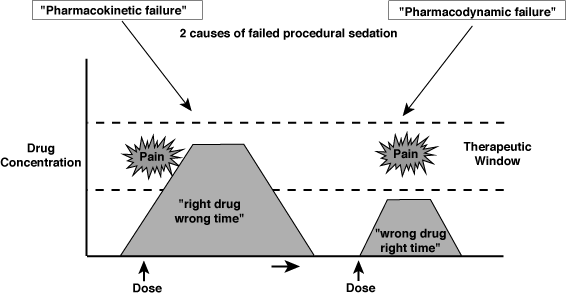
In the figure below two examples of inadequate procedural sedation are shown. The first, pharmacokinetic failure, is an example of giving an analgesic “right drug” at the wrong time, such that the peak analgesic effect occurs after the painful procedure. A classic example is performing a fracture reduction immediately after intravenous morphine (a slow acting analgesic). The second example is one of pharmacodynamic failure in which an inappropriate drug (i.e. a drug without analgesic properties) is given for a painful procedure.
The Procedure
Defining the characteristics of the procedure assists in determining the most appropriate sedative agent. Based on the degree of procedural pain and amount of procedural immobility (stillness needed to complete the procedure), four categories of procedures can be defined. The box below describes the four categories in terms of desired clinical effects, types of procedures, and appropriate sedative medications.
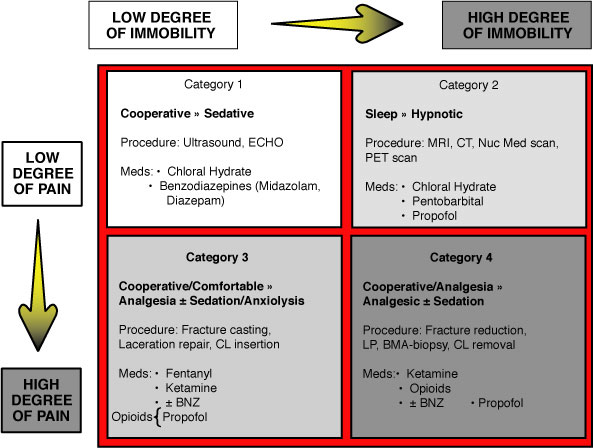
- Painful (Invasive) Procedures: e.g. Heme/Onc procedures: Lumbar Punctures, Bone Marrow biopsies
- Invasive procedures are considered one of the most stressful parts of pediatric cancer treatment
- Initial poorly controlled procedural pain is associated with the development of anxiety related disorders in children and diminished analgesic effects in subsequent procedures.
- The American Academy of Pediatrics (AAP) recommends that procedural pain management be part of the “front line” treatment in children with cancer (Pediatrics 1990; 86:826)
- “Make sure that pain is treated promptly and adequately” the #1 request from a Parent’s Wish List for interactions with their children’s doctors.
- Non-Painful (Non-Invasive) Procedures:
- Non-invasive diagnostic studies that require a high level of patient “immobility” to be satisfactorily completed and interpretable include BAERs, MRIs, CTs, PETs, and Nuclear Medicine scans. These studies often require Moderate to Deep Sedation.
- ECHOs and Ultrasounds typically require less immobility and consequently a lower level of sedation