Anxiolytic Drugs
These are drugs that are used to make children less anxious or more comfortable. These are not agents that will induce “sleep.” They are particularly effective for noninvasive procedures or slightly painful procedures that do not require a high degree of stillness. They are commonly used as adjuncts with analgesic agents for procedures that are painful and require varying degrees of stillness.
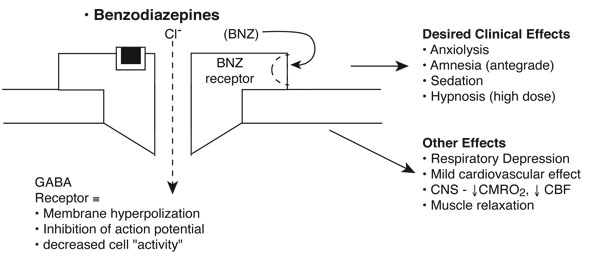
Benzodiazepines (BNZs): Diazepam, Midazolam, Lorazepam
Pharmacodynamic Features
Desired clinical effects
Benzodiazepines are commonly used in procedures associated with mild to moderate discomfort not requiring high levels of immobility (Manso 2019). These are not reliable hypnotic agents, and they are especially useful as adjuncts with analgesics. Antegrade amnesia is one of the most important clinical effects of BNZs, particularly in children undergoing invasive procedures.
Benzodiazepines work by enhancing gamma-aminobutyric acid (GABA) neurotransmission by binding to specific BNZ receptors on the GABA-A receptor complex. They enhance chloride flux across ligand-gated chloride channels, resulting in membrane hyperpolarization and inhibition of the action potential.
Other effects
- Respiratory
- Respiratory depressive effect, especially when administered with other agents that have similar impact on respiratory drive
- Reduces airway tone
- Cardiovascular
- Mild myocardial depressive effect
- Neurological
- Reduces cerebral blood flow
- Increases seizure threshold
- Other
- Anti-nausea
Pharmacokinetic Properties
The time of clinical onset and duration of action following single-dose administration is primarily related to lipid solubility.
| Drug | Lipid Solubility* | Volume of Distribution | Clearance (mL/kg/min) | Elimination half-life (hours) | Metabolism | Active Metabolite |
|---|---|---|---|---|---|---|
| Diazepam | +++ | 0.7-1.7 | 0.2-0.5 | <20-40 >40 (critically ill) |
Oxidation ↓ Conjugation |
Desmethyldiazepam (t 1/2 β=80-70 hours) |
| Midazolam | +++ | 1.1-1.7 | 6.4-11.1 | 2-4 >6-10 (critically ill) |
Oxidation ↓ Conjugation |
α-Hydroxymidazolam (t 1/2 β=1 hour) |
| Lorazepam | + | 1.1-1.8 | 0.7-1.0 | 10-20 >40 (neonates) |
Conjugation | – |
*Lipid Solubility: (+) low, (++) medium, (+++) moderate, (++++) high
- Diazepam and midazolam have active metabolites and require both oxidation and conjugation.
- Lorazepam does not have metabolites and requires only conjugation.
- All BNZs are highly protein-bound.
| Benzodiazepine | Dose | Repeat Dose | Onset | Duration |
|---|---|---|---|---|
| Diazepam | 0.1-0.15 mg/kg | 0.05-0.1 mg/kg q 3-5 min |
<60 sec | 15-30 min |
| Midazolam | 0.05-0.1 mg/kg | 0.05 mg/kg q 3-5 min |
<60 sec | 15-30 min |
| Lorazepam | 0.05 mg/kg | .025-.05 mg/kg q 10-15 min |
2-3 min | 1-2 hrs |
| Administer | Dose | Clinical Onset | (+) | (-) |
|---|---|---|---|---|
| Intranasal | 0.2-0.4 mg/kg | 10-15 min | faster effects | irritating to nasal mucosa |
| Rectal | 0.3-0.7 mg/kg | 15-20 min | dependable | not older children |
| Oral | 0.3-0.7 mg/kg | 20-30 min | easy admin | variable onset, bad taste |
Nitrous Oxide
Pharmacodynamic Features
Desired Clinical Effects
Nitrous oxide is a colorless, odorless gas that imparts anxiolysis and analgesia for mildly painful procedures, such as venipuncture or urinary catheter insertion. With a Minimum Alveolar Concentration (MAC) of 100-110%, it is considered a weak anesthetic gas and should thereby not be used as a sole agent during very painful or invasive procedures. However, its pharmacodynamic profile renders it a useful agent to be paired with anxiolytics such as benzodiazepines.
Other Clinical Effects
- Respiratory
- Diffusion hypoxia — When the patient stops inhaling nitrous oxide, the concentration gradient favors movement of nitrous oxide from blood into alveolus, thereby diluting out oxygen from this space. This results in a phenomenon called “diffusion hypoxia.” This is mitigated with administration of supplementation oxygen for several minutes following cessation of nitrous oxide administration.
- Mild respiratory depression – dose-dependent depression of ventilatory response to hypoxemia
- Cardiovascular
- Minimal impact on healthy myocardium
- Vasoconstrictive response and effect on patients with pulmonary hypertension
- Hematologic + Neurologic
- Inactivation of methionine synthetase, with prolonged exposure can lead to functional Vitamin B12 deficiency (altered bone marrow function and peripheral neuropathy)
- Other
- Gas-filled spaces — nitrous oxide diffuses into gas-filled spaces much more efficiently than nitrogen can diffuse out, thereby increasing the volume and pressure in these spaces. This has important implications for conditions such as pneumothorax, bowel obstruction, and pneumocephalus
- Nausea and vomiting
Pharmacokinetic Features
Nitrous oxide’s low solubility in both blood and fat imparts a very fast onset and short duration of action upon discontinuation. The result of this is that it takes effect almost immediately upon administration. A full 3 minutes of administration is required to achieve the maximum effect.
Once inhaled, nitrous oxide is not metabolized by the body in any meaningful way, but it is exhaled unchanged. Central nervous system effects of the gas dissipate quickly as it travels down its concentration gradient into the alveolus and then out of the body.
Basics
Clinical Use: Nitrous oxide is very useful for mild or moderately invasive procedures, either alone or paired with benzodiazepines (Tobias 2013).
If you are interested in learning more about nitrous oxide, including information about how to incorporate nitrous oxide as a tool in your clinical practice, consider joining us for a hands-on instructional course! The University of Wisconsin Pediatric Sedation Program offers an annual course to impart practical skills and information about nitrous oxide sedation.
Hypnotic Drugs (“Sleepers”)
These are drugs that are used to make children sleep. These agents are particularly effective for procedures requiring a high level of immobility.
Propofol: Potent Sedative-Hypnotic
Pharmacodynamic Features
Desired Clinical Effects
Propofol is a gabaminergic agent that is universally reliable and effective at inducing deep sedation in all patients, without inducing drug tolerance. It has been successfully utilized for procedures that require a high degree of immobility, though because it has no analgesic properties, it should be paired with analgesic agents when used during painful or invasive procedures. It has important safety considerations especially pertaining to the respiratory and cardiovascular system, and it should only be used by clinicians skilled in pediatric procedural sedation. Institutional recommendations with regard to privileging and credentialing vary by state.
Other Clinical Effects
- Respiratory: moderate-to-severe respiratory depressant effects
- Blunted/erratic airway reflexes
- Blunted ventilatory response to hypercarbia/acidosis and to hypoxemia
- Reduced upper airway tone
- Cardiovascular:
- Systemic hypotension due to vasodilation and overall reduced sympathetic tone
- Mild myocardial depression with reduced inotropy
- Other: anti-emetic, anti-epileptic properties. Has tendency to induce warm sensation at the IV site on administration
Pharmacokinetic Features
Propofol is metabolized by the liver by and excreted by the kidneys.
Basics
- Dose:
- Bolus: varies by patient age
- Induction dose = 1-2 mg/kg/min (average 3-4 mg/kg) until sleeping (higher doses in younger children)
- Titration dose = 0.5-1 mg/kg bolus
- Infusions: dose-dependent clinical effects
- 25 mkm = antiemetic
- 75 mkm = amnestic
- 100 mkm = hypnotic
- 250 mkm = max dose to maintain adequate spontaneous ventilation
- Bolus: varies by patient age
- Induction time: onset within 30 seconds, peaks 1-2 minutes
- Recovery time: 7-10 min for single bolus, 20 minutes for infusion
- Effectiveness: universally effective to induce deep sedation
Clinical Use
Its rapid onset and short duration render it a very useful medication for nonpainful procedures requiring sedation or sleep (e.g., MRI, CT, nuclear medicine studies). Propofol is the most common sedative used by institutional members of the Pediatric Sedation Research Consortium, the research arm of the Society for Pediatric Sedation.
Propofol is often paired with an analgesic agent to induce deep sedation during painful procedures, including lumbar puncture and bone marrow biopsy.
Brief procedures can be facilitated using intermittent bolus dosing of propofol, whereas prolonged procedures such as MRI are better facilitated with a continuous infusion.
Dexmedetomidine: Potent Sedative-Hypnotic with mild Analgesic Properties
Pharmacodynamic Features
Desired Clinical Effects
Dexmedetomidine is a selective central alpha-2 agonist with effects in the central nervous system, the peripheral nervous system, and the autonomic ganglia. It produces sedation, anxiolysis, and analgesia. It is a useful agent for supporting patients during procedures that require a moderate- to high-degree of immobility, especially imaging procedures.
Other Clinical Effects
- Respiratory: Minimal respiratory depressant effects.
- Minor-to-no impact on upper airway tone.
- Preserved ventilatory responses to hypoxemia/hypercarbia
- Cardiovascular:
- Slow cardiac conduction at the SA and AV node, resulting in common side effect of bradycardia
- Initial increase in systemic vascular resistance
- Lower blood pressures secondary to reduced heart rate
- Neurologic:
- resembles non-REM sleep on EEG monitoring
- Other:
- May be neuroprotective against the development of neuroapoptosis of neurocognitive impairment, at least in animal models
Pharmacokinetic Features
Dexmedetomidine is metabolized in the liver by glucuronidation and by P450 pathways to inactive metabolites. May have prolonged effect in patients with renal failure owing to reduced protein binding in this patient population.
Basics: Generally Safe and Effective
- Dose:
- Bolus 1-2 mcg/kg IV for induction. Should be administered over 5-10 minutes
- Infusion 1-3 mcg/kg/hr
- Can also be administered via a variety of routes of administration, including intranasal, buccal, and intramuscular routes.
- Repeat dose: 1–2 mcg/kg IV
- Induction time: takes effect within 5 minutes, peak effect 20 minutes
- Recovery time: 1 hours
- Elimination t 1/2 = 2-3 hours
| Administer | Dose | Repeat Dose | Onset | Duration |
|---|---|---|---|---|
| IV | 1-2 mcg/kg delivered over 5-10 minutes |
0.0-15 mcg/kg every 10 min |
10 min | 1-2 hrs |
| IN | 2-4 mcg/kg (max 200 mcg) |
— | 20-35 min | 2 hrs |
| IM | 1-4 mcg/kg | 1-2 mcg/kg | 10 min | – |
| PO | 1-4 mcg/kg | — | 45 min | — |
Clinical Use: very effective for non-painful procedures requiring high level of immobility. Dexmedetomidine takes longer to reach clinical effect (and takes longer to wear off) than propofol does, which makes it a less efficient choice for inducing and maintaining deep sedation. However, its safety profile makes it especially useful in patients for whom propofol would pose additional risk (i.e., patients with symptoms of upper respiratory infection or patients with sleep-disordered breathing).
Dexmedetomidine also is an agent that can be delivered via multiple routes of administration, which can be a desirable feature especially for children who do not require IV access for their procedure (i.e., non-contrast MRI or CT).
Analgesic Drugs
These are drugs used for painful procedures.
Nonsteroidal Anti-Inflammatory Agents (Not a Sedative)
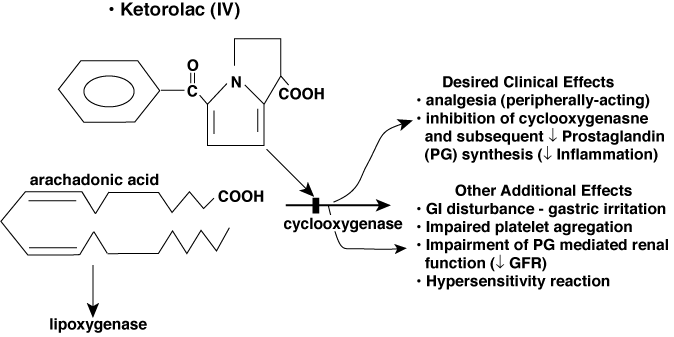
Use:
- Postoperative pain in children.
| Reaction to Treatment | Morphine (n=48) | Ketorolac (n=54) |
|---|---|---|
| Pain relief in first hour | 27 (56%) | 31 (57%) |
| Pain relief in first 2 hrs | 28 (58%) | 37 (68%) |
| Maximum pain relief in first 2 hrs | 21 (44%) | 27 (50%) |
| Required remedication within the first 4 hrs | 30 (63%) | 31 (58%) |
| Treatment failure (never achieved pain relief) | 7 (14%) | 2 (3.7%) |
Opioid Agonists
Morphine, Fentanyl, Meperidine, Methadone, Hydromorphone, Codeine, Alfentanil
Pharmacodynamic Features
Opioid agonists bind to specific opioid receptors (primarily µ receptors) distributed throughout the neuraxis. Opioids inhibit spontaneous neuronal firing and excitatory neurotransmitter release.
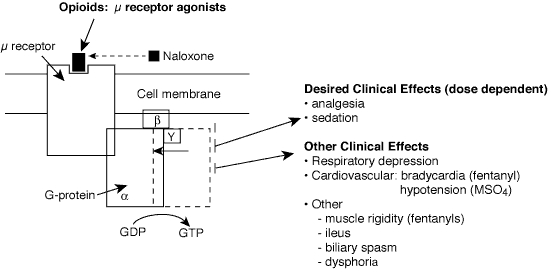
Basics
Clinical onset and duration distinguish morphine from fentanyl, although they have similar half-lives.
| Opioid Agonist | Lipid Solubility* | Protein Binding (%) | Volume of distribution (L/kg) | Clearance (mL/kg/min) | Elimination half-life (hours) | Active Metabolites |
|---|---|---|---|---|---|---|
| Morphine | + | 30% (adults, children)
18-22% (neonates) |
3.2-4.7 | 12.4-15.2 | 2-4 (adults, children), 6-7 (infants) | Morphine-3-glucuronide (excitatory)
Morphine-6-glucuronide (active) |
| Fentanyl | ++++ | 80%-85% | 3.2-4.2 | 11.2-13.3 | 2.7 | – |
| Alfentanil | +++ | 92% | 0.86 | 6.4 | 1-1.6 | – |
| Methadone | +++ | 80%-85% | 7.1 | 5.4 | 15-30 | – |
*Lipid Solubility: (+) low, (++) medium, (+++) moderate, (++++) high
Notes:
- Morphine is much less protein-bound than fentanyl.
- Fentanyl does not have active metabolites.
- Morphine and fentanyl have similar elimination half-lives and much different lipid solubility.
| Opioid Agonist | Dose | Repeat Dose | Onset | Duration |
|---|---|---|---|---|
| Morphine | 0.05-0.2 mg/kg | 0.05 mg/kg q 10 min |
5-10 min | 3-4 hours |
| Fentanyl | *0.5-2 µg/kg | 0.5 mg/kg q 2-3 min |
2-3 min | 30-60 min |
*Administer over ~2 min
Clinical Use
Procedures associated with moderate to severe discomfort (pain). Benzodiazepines augment opioid effects and provide amnesia. Opioids pair well with sedative-hypnotic agents such as propofol for procedures that require a high degree of stillness.
Ketamine
Phencyclidine derivative with dissociative sedative, analgesic and amnestic properties.
Pharmacokinetic Features
Ketamine noncompetitively blocks the N-methyl-D aspartate (NMDA) receptor, part of a class of glutamate receptors mediating excitatory neurotransmission. Ion fluxes and subsequent excitatory neurotransmission are inhibited.
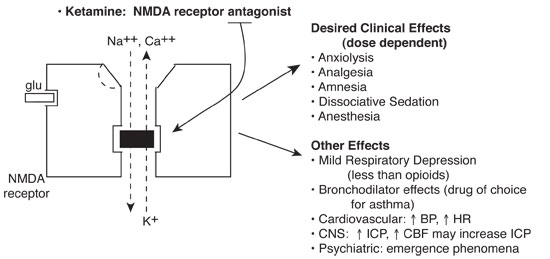
Basics
Clinically effective by a number of different routes. Administer with benzodiazepines to attenuate neuropsychiatric effects and antisialagogue to reduce secretions. Ketamine is relatively contraindicated in any child with perceptual problems (visual, auditory, psychiatric). The single most severe adverse effect occurring in a healthy child is laryngospasm.
| NMDA Receptor Antagonist | Protein Binding (%) | Volume of distribution (L/kg) | Clearance (mL/kg/min) | Elimination half-life (hours) | Active Metabolites |
|---|---|---|---|---|---|
| Ketamine | 12% | 3 | 18 | 2-3 | Norketamine* |
*Predominates after enteral administration
| Administer | Dose | Repeat Dose | Clinical Onset | Clinical Peak | Clinical Duration |
|---|---|---|---|---|---|
| IV | 0.5-1 mg/kg* | 0.5 mg/kg (every 2-3 min) |
<60 sec | 1-2 min | 10-15 min |
| IM | 2-4 mg/kg | 2-4 mg/kg | 1-2 min | 2-4 min | 30-60 min |
| Oral | 6-10 mg/kg** | 6-10 mg/kg | ~10-15 min (variable) |
~20-45 min | 2-3 hours |
| Rectal | 6-8 mg/kg** | 6-8 mg/kg | ~5-10 min (variable) |
10-20 min | 2-3 hours |
*Midazolam 0.1 mg/kg (IV)
**Midazolam 0.2-0.3 mg/kg (p.o., p.v.)
Clinical Use
Procedures associated with moderate to severe discomfort and pain. Avoid in individuals with intracranial hypertension, systemic hypertension, or neuropsychiatric disorders and any child with visual or auditory problems (perceptual disorders) (Tobias 1992, Parker 1997, Kennedy 1998).
Reversal Agents
Flumazenil
Flumazenil is a short-acting agent that reverses benzodiazepine-induced sedation. Re-sedation may occur due to its short duration of action, therefore additional doses may be necessary. Flumazenil is not useful for barbiturate- or opioid-induced sedation.
Basics
- Dose: 0.01 mg/kg IV (max. dose 0.2 mg) If desired level of consciousness is not obtained after waiting an additional 45 seconds, give repeat dose.
- Repeat dose: 0.005–0.01 mg/kg IV
- Induction time: 1–3 min (peak effect 6–10 min)
- Duration of effect: Usually less than 60 minutes. Duration is related to the dose given and the benzodiazepine plasma concentrations; reversal effects of flumazenil may be shorter than the effects of the benzodiazepine.
Nursing Considerations
Re-sedation may occur because the duration of effect of the benzodiazepine may exceed that of flumazenil. In the event of re-sedation, repeat doses may be administered at 20-minute intervals as needed.
Precautions
- Should not be given to patients who are on benzodiazepines as part of therapy for a seizure disorder.
- Give cautiously to patients who are on medications known to lower the seizure threshold, such as tricyclic antidepressants, theophylline, isoniazid or lithium. Use of flumazenil in these patients could precipitate a seizure.
Naloxone
Naloxone is a short-acting agent that reverses opioid-induced sedation. It competes and displaces opioids at opioid-receptor sites. Re-sedation may occur due to its short duration of action, therefore repeated doses are usually needed. Naloxone is not useful for barbiturate-, benzodiazepine- or phencyclidine-induced sedation.
Basics
- Dose: 0.01 mg/kg (IV) over 30 sec as undiluted preparation
- Repeat dose: 0.01 mg/kg IV may be repeated every 2–3 min as needed based on response
- Induction time: Within 2 min
- Duration of effect: 20–60 min. Duration is shorter than that of most opiates, therefore repeated doses are usually needed.
Clinical Effects
- Re-sedation may occur, because the duration of effect of the opiate may exceed that of naloxone. In the event of re-sedation, repeat doses may be administered.
- Naloxone may improve alertness but should not be substituted for an adequate period of postprocedure monitoring. Monitoring (including blood pressure) must continue until the child returns to and maintains his or her baseline level of consciousness.
- Naloxone may precipitate withdrawal symptoms (hypertension, sweating, agitation, irritability, shrill cry, failure to feed) in opioid-dependent children. Use cautiously in children on opioid drips.
References
Manso MA, Guittet C, Vandenhende F, Granier LA. Efficacy of oral midazolam for minimal and moderate sedation in pediatric patients: A systematic review. Paediatr Anaesth. 2019 Nov; 29(11):1094-1106.
Tobias JD. Applications of nitrous oxide for procedural sedation in the pediatric population. Pediatr Emerg Care. 2013 Feb;29(2):245-65.
Tobias JD, Phipps S, Smith B, Mulhern RK. Oral ketamine premedication to alleviate the distress of invasive procedures in pediatric oncology patients. Pediatrics. 1992 Oct;90(4):537-41. PMID: 1408506.
Parker RI, Mahan RA, Giugliano D, Parker MM. Efficacy and safety of intravenous midazolam and ketamine as sedation for therapeutic and diagnostic procedures in children. Pediatrics. 1997 Mar;99(3):427-31. doi: 10.1542/peds.99.3.427. PMID: 9041300.
Kennedy RM, Porter FL, Miller JP, Jaffe DM. Comparison of fentanyl/midazolam with ketamine/midazolam for pediatric orthopedic emergencies. Pediatrics. 1998 Oct;102(4 Pt 1):956-63. doi: 10.1542/peds.102.4.956. PMID: 9755272.