Like most children, five-year-old Lexi Frye will likely have a viral upper respiratory infection (URI) at least once this year. She may suffer though sniffles, cough, and congestion—and then recover. Or, she may be one of the estimated eight percent of children who develop acute bacterial sinusitis.
Although Lexi isn’t sick now, a study nurse is obtaining a nasopharygeal (NP) swab sample from her as part of a five-year clinical study led by department chair Ellen Wald, MD, that aims to determine the incidence and predictors of this common complication.
The Path From Viral Infection to Sinusitis
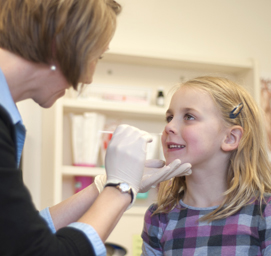
Lexi is one of approximately 80 participants to date in Dr. Wald’s study, which launched in December 2011. Children between four and seven years old enroll when they’re healthy, and participate for a full year.
At the initial visit, a study nurse obtains an NP swab sample from the child. The child also provides a nasal mucus sample.
Then, if the child develops viral URI symptoms that last for over 48 hours, a nurse obtains another NP swab sample—usually at a home visit. The nurse also follows up by phone every three days; on day 10, if symptoms are improving, the child returns for a recovery visit and final NP swab.
If symptoms are not improving, the child is referred to his or her primary care provider to determine whether acute bacterial sinusitis is present.
The process is repeated each time the child has a symptomatic viral URI. Participants also provide NP samples at four surveillance visits throughout the year. All samples are analyzed and identified using high-throughput multiplex PCR-based viral diagnostics.
“Our primary goals are to determine the incidence of acute bacterial sinusitis and identify the types of viral infections and other risk factors that predispose to it,” Dr. Wald explained.
“Better understanding those predictive factors will allow us to develop appropriate prevention and treatment strategies to reduce the burden of disease and antimicrobial resistance.”
Clinical Research Team ‘Goes the Extra Mile’
A study of this size and scope requires substantial staff support. That’s where the department’s clinical research team, led by Stacey Moyer, MSN, RN, comes in.
In addition to Moyer, the team includes four nurses (Jody Belling, RN, PNP; Andrea Blom, RN, BSN; Patricia Filas-Mortensen, RN, MSN; and Kirstin Carlson-Dakes, RN, MEd), and one financial specialist (Cherie Schommer).
Together, they supported 43 studies across all divisions in FY12. Services range from Institutional Review Board (IRB) application review, budget administration, patient recruitment, and overall study coordination. They also provide guidance for faculty who are just beginning to develop a study protocol.
Moyer explained that a dedicated departmental team can help investigators demonstrate capacity when seeking clinical research opportunities. It’s also a way to cultivate rapport with sponsors, who are continually seeking study sites.
But for Moyer and her colleagues, who get to know participating children through home, daycare, and clinic visits, it’s the personal interactions that are particularly gratifying.
“At home visits, children show us what they bought with the money they receive for study participation,” she recalls. “Families are so grateful we’re able to make those visits, so their children don’t have to come to the clinic for each follow-up.”
“That’s what makes our team unique. We can go the extra mile.”
“Sinusitis in Children and the Nasopharyngeal Microbiome,”
(PI: Ellen Wald, MD; #NCT01492868) is funded by the National Institutes
of Health’s National Institute of Allergy and Infectious Diseases.