The major focus of the Smith Research Group is an intracellular stress response originating from the endoplasmic reticulum (ER) called the “Unfolded Protein Response” or “UPR”. The UPR has been implicated in such diverse processes as viral infection, cancer, neurodegenerative disease, autoimmune diseases, diabetes and cardiovascular disease. We have been most interested in understanding the immune consequences of UPR activation, specifically as it impacts inflammatory cytokine production. Macrophages undergoing a UPR produce greatly augmented type I IFN, TNF-α and IL-23 in response to infectious stimuli. The many interactions between UPR and immune signaling pathways and the resulting enhancement of immune responses suggest the UPR may serve as a “danger signal” immune amplifier.
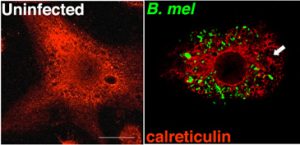 Infectious agents, particularly those that invade cells to replicate, have developed multiple ways of dealing with the host UPR. The manipulation of host UPR by viral infections has been widely explored and continued to yield interesting surprises. Much less is known about how bacteria interface with the UPR. We have found that one human pathogen, Brucella species induces a massive disruption of ER structure (imaged by anti-calreticulin staining in red and B. melitensis in green in the figure) and triggers a full UPR. The UPR may affect multiple aspects of pathogenicity, including cytokine production, bacterial replication, host cell apoptosis, antigen presentation and memory. Currently it is not clear how Brucella manipulates the UPR to benefit infection while avoiding the negative repercussions of ER stress.
Infectious agents, particularly those that invade cells to replicate, have developed multiple ways of dealing with the host UPR. The manipulation of host UPR by viral infections has been widely explored and continued to yield interesting surprises. Much less is known about how bacteria interface with the UPR. We have found that one human pathogen, Brucella species induces a massive disruption of ER structure (imaged by anti-calreticulin staining in red and B. melitensis in green in the figure) and triggers a full UPR. The UPR may affect multiple aspects of pathogenicity, including cytokine production, bacterial replication, host cell apoptosis, antigen presentation and memory. Currently it is not clear how Brucella manipulates the UPR to benefit infection while avoiding the negative repercussions of ER stress.
In my lab we have been investigating the intersection of UPR stress response and immune function through the lens of the host-pathogen interaction, using this model organism and others.
Research News

Department supports 15 research and scholarly projects in 2023 with its R&D Awards
In calendar year 2023, the Department of Pediatrics provided department members with $142,105 through its Research and Development (R&D) Awards. The purpose of the R&D Awards fund is to support research and scholarly activities, including …
December 7, 2023
Judy Smith awarded grant from Merck
Congratulations to principal investigator Judy Smith, MD, PhD, associate professor, Allergy, Immunology, and Rheumatology, who recently received a one-year grant from Merck in the amount of $203,983. Her proposal “Epithelial Determinants of Respiratory Syncytial Virus Immune …
August 16, 2022
Four pediatrics faculty advisors, students receive Hilldale research fellowships
Congratulations to the following faculty and students who were recently awarded Hilldale Faculty/Undergraduate Research Fellowships for the 2022-2023 academic year. The fellowship provides research training and support to undergraduate students, and it provides them the …
July 15, 2022
Judy Smith receives R01 renewal for Brucella study
Congratulations to Judy Smith, MD, PhD, associate professor, Allergy, Immunology, and Rheumatology, who will serve as Co-Investigator on a renewal subaward with Principal Investigator Sergio Oliveira, DVM, PhD, of the Federal University of Minas Gerais and …
December 10, 2021
Judy Smith awarded R21 from NIAID
Congratulations to Judy Smith, MD, PhD, associate professor, Allergy, Immunology, and Rheumatology, on her recently awarded R21 from the National Institutes of Health/National Institute of Allergy and Infectious Diseases for her project, “Unfolded protein response (UPR) determinants …
October 12, 2021- More News...
