Immunotherapy is the process of using a component, or components, of the immune system to eliminate cancer.
University of Wisconsin and Immunotherapy Research for Childhood Cancer
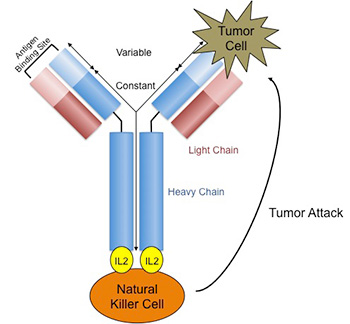 Because of advancements in genetic engineering and in culturing cells, we can now produce more sophisticated immunotherapies that are more specific for pediatric cancer. At UW, research by Dr. Paul Sondel contributed toward the development of a monoclonal antibody against neuroblastoma, the most common solid tumor in children outside of the brain. This antibody, called ch14.18 or dinutuximab, binds to neuroblastoma and makes it a target by natural killer (NK) cells for destruction. By doing so, in phase III trials we observed an improvement in survival in children with stage IV neuroblastoma, the most advanced form of the cancer. Dinutuximab was FDA approved in 2015. Other similar antibodies are also being tested at UW, like the immunocytokine hu14.18-IL2 (see figure).
Because of advancements in genetic engineering and in culturing cells, we can now produce more sophisticated immunotherapies that are more specific for pediatric cancer. At UW, research by Dr. Paul Sondel contributed toward the development of a monoclonal antibody against neuroblastoma, the most common solid tumor in children outside of the brain. This antibody, called ch14.18 or dinutuximab, binds to neuroblastoma and makes it a target by natural killer (NK) cells for destruction. By doing so, in phase III trials we observed an improvement in survival in children with stage IV neuroblastoma, the most advanced form of the cancer. Dinutuximab was FDA approved in 2015. Other similar antibodies are also being tested at UW, like the immunocytokine hu14.18-IL2 (see figure).
The Capitini Laboratory and Immunotherapy
Dr. Christian Capitini is simultaneously performing preclinical work studying ways to improve the efficacy of combining hu14.18-IL2 and NK cells in neuroblastoma after bone marrow transplant. In fact, they have already developed a novel way of tracking the infused NK cells using fluorine-19 MRI, an approach they would like to bring to the clinic in the coming years.
In 2013, UW was selected as one of 8 children’s hospitals as part of a Pediatric Cancer Dream Team. The goal of this team is to (1) genetically sequence pediatric cancers to look for new targets on the surface of cancer cells that can be recognized by the immune system, and (2) to develop novel immunotherapies against these targets for the clinic. As part of this effort, Dr. Capitini was a site-Principal Investigator on multi-center clinical trials testing the efficacy of chimeric antigen receptor (CAR) T cells (called tisagenlecleucel) for childhood B cell acute lymphoblastic leukemia (ALL). Because of these efforts, Tisagenlecleucel was FDA approved in 2017 as the first gene therapy and cellular immunotherapy for cancer. UW is one of a limited number of hospitals to offer this therapy.
Ongoing work is examining the usage of CRISPR/Cas9 to genetically modify T cells, NK cells and macrophages to express a CAR without the use of viruses. These virus-free CRISPR CAR T cells are being studied for a variety of pediatric tumors like neuroblastoma, osteosarcoma and brain tumors alone and in combination with radiopharmaceutical therapy. Additional innovative work is being done by Dr. Sondel and Dr. Richards as part of a St. Baldricks’s Foundation Empowering Pediatric Immunotherapies for Childhood Cancer (EPICC) Team. To hear more about immunotherapy research and teaching by Dr. Capitini, please refer to the following videos:
Introduction to Immunology for the non-Immunologist
CAR T cell therapy approved for children and young adults with leukemia
University of Wisconsin picked as a site to provide promising and expensive cancer drug
Kymriah’s journey from lab to lifesaver: the phase I trial (Part 1)
Kymriah’s journey from lab to lifesaver: the phase I trial (Part 2)
Adoptive Cell Therapies for Pediatric Cancers: Merging Preclinical and Clinical Studies