Our research focuses on understanding the basic molecular mechanisms that regulate cell movement—in the context of wound healing, inflammation and cancer. Cell migration plays a central role in many different disease processes including cancer, heart disease and autoimmune disease. Insight into the mechanisms that regulate cell migration will contribute to our understanding of basic cellular processes, but will also aid in the identification of new treatment strategies for a wide variety of medical conditions. Despite extensive interest in the receptors and mechanisms involved during cell migration, many fundamental questions remain unanswered. What are the mechanisms by which a cell initiates and then subsequently stops directional cell migration? How are intracellular signaling events coordinated both temporally and spatially to promote productive, directional cell movements? What are the mechanisms that regulate the migration of leukocytes into areas of inflammation? How do tumor cells invade and metastasize?
Our research is aimed at understanding the cellular and molecular mechanisms that regulate cell migration using biochemical genetic and imaging approaches. We use state of the art live imaging, biosensors and photomanipulation to examine and control polarity of cell signaling during cell migration in live animals. We also examine host-pathogen interactions in zebrafish.
Current Projects
- Using zebrafish to model primary immune deficiency and chronic inflammatory disease
- Innate immune response in patients with lupus
- Imaging leukocyte migration and intracellular signaling in vivo
- Tumor invasion and metastasis: understanding invasive migration
- Host pathogen interactions in zebrafish
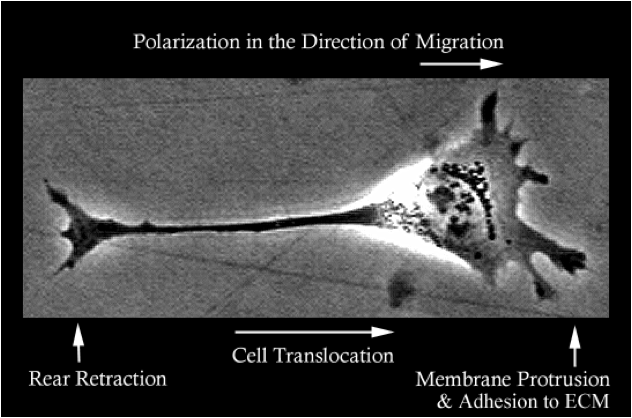
Cell Translocation
The Huttenlocher lab studies the basic molecular mechanisms that regulate cell migration and is interested in how defects in cell migration contribute to human disease. To migrate cells extend leading edge protrusions, stabilize adhesions and subsequently detach from the cell’s rear. We are interested in how adhesions are regulated at both the leading and trailing edge of migrating mesenchymal and amoeboid cells.
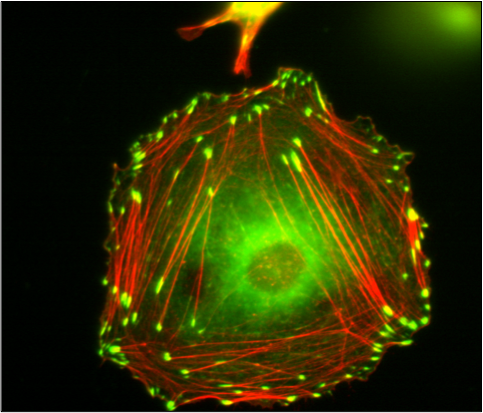
Vinculin Containing Focal Adhesions and Actin Stress Fibers in a Fibroblast-like Cell
The picture shows vinculin (green) containing focal adhesions and actin stress fibers (red) in a fibroblast like cell. The Huttenlocher laboratory studies how adhesion dynamics are regulated. We are interested in the dynamics of both focal adhesions and degradative structures including podosomes and invadopodia.

Zebrafish
The Huttenlocher laboratory uses zebrafish to study cell migration and inflammation in vivo.
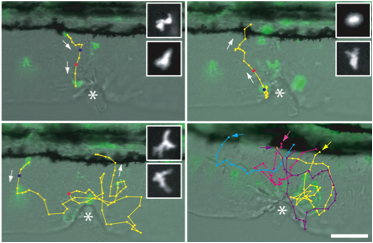
Tissue Wounding
Tissue wounding induces the recruitment of neutrophils to the wound and subsequent reverse migration back toward the vasculature. Cell tracks show live imaging of GFP positive neutrophils from the vasculture to the wound and back toward the vasculature.
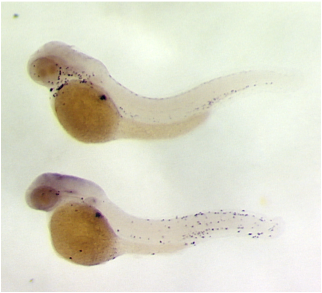
Genetic Screens for Zebrafish Mutants
We have performed genetic screens for zebrafish mutants that exhibit chronic inflammation and infiltration of immune cells into the epidermis. Shown here in situ for zebrafish neutrophil myeloperoxidase. The bottom panel shows a zebrafish line identified in a screen for chronic inflammation mutants.
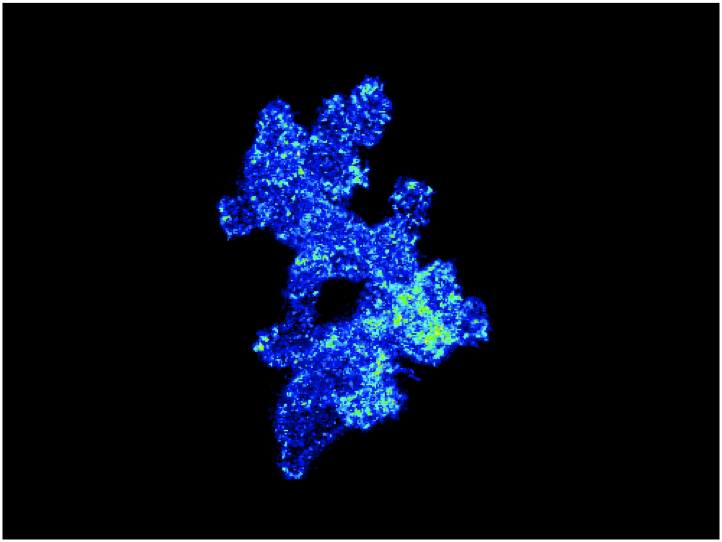
Understanding How Cell Signaling is Regulated During Chemotaxis of Leukocytes in Vivo
A major focus for the laboratory is to understand how cell signaling is regulated during chemotaxis of leukocytes in vivo. The image shows an overlay of sequential ratiometric images to show the polarization of PI3K signaling as neutrophils are migrating in vivo.
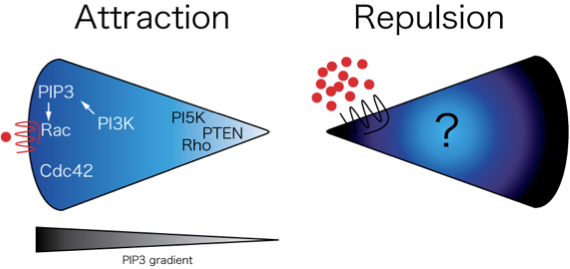
Reverse Migration of Neutrophils from Tissues
A major challenge for our research group is to understand the mechanisms that regulate bidirectional trafficking of leukocytes to and from inflamed tissue in vivo. We observed reverse migration of neutrophils from tissues back toward to vasculature to resolve inflammatory responses. We are now studying the mechanisms that regulate this reverse migration.
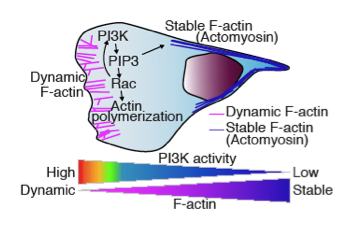
Cell Signaling Neutrophil Neutrophil Chemotaxis in Vivo
The schematic shows cell signaling neutrophil neutrophil chemotaxis in vivo. We have observed dynamic F-actin at the leading edge and more stabilized F-actin at the uropod (Yoo, Developmental Cell, 2010).
Research News

Physician Scientist Training Pathway supports new residents who wish to combine medicine and scientific research
Every year when new students enter the UW School of Medicine and Public Health (SMPH), some combine their medical studies with scientific research, continuing often already extensive undergraduate research experience. Some choose to look for …
May 8, 2024
Tanner Robertson’s zebrafish image named one of 12 winners in campus Cool Science Image Contest
An image of a transparent zebrafish’s lymphatic network captured with a Zeiss spinning disk confocal microscope earned microbiologist Tanner Robertson, PhD, recognition in UW–Madison’s 13th annual Cool Science Image Contest. Robertson is a post-doc researcher in the …
October 13, 2023
Anna Huttenlocher publishes book about her father and his discovery of synaptic pruning
What is a sabbatical for? Travel, study, time away from the usual professional routine spent in ways that may enhance one’s work upon return — that is the norm. Anna Huttenlocher, MD, is a pediatric …
August 30, 2023
Novel non-mammalian adaptive immune system function revealed in study by post-doc researcher in Huttenlocher Research Group
An investigation using zebrafish genetically developed to remain transparent into adulthood by the laboratory of Anna Huttenlocher, MD, professor in the Division of Allergy, Immunology, and Rheumatology and in the Department of Medical Microbiology and …
July 24, 2023
Scientist, clinician, and mentor: Anna Huttenlocher wins 2022 Hilldale Award in Biological Sciences
Since 1987, four University of Wisconsin–Madison faculty members have been honored for their substantial contributions to research, teaching and service with the prestigious Hilldale Award. The 2022 winner in the biological sciences is Anna Huttenlocher, …
April 13, 2022- More News...

Professor
huttenlocher@wisc.edu