Welcome to the University of Wisconsin Department of Pediatrics
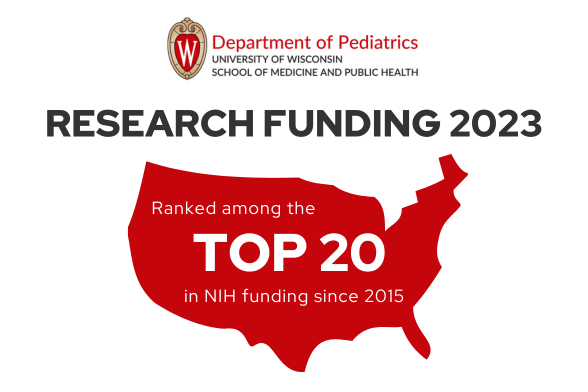
The Department of Pediatrics at the University of Wisconsin School of Medicine and Public Health promotes and enhances the health of children through outstanding clinical care, exemplary education of pediatric trainees, performance of cutting-edge research, and vigorous advocacy. It is the academic home to more than 200 faculty members across 16 subspeciality divisions.
Train with us
Formal training programs for medical students, residents, fellows, postgraduate researchers, and faculty provide subspecialty expertise to prepare the next generation of clinicians, scientists, scholars, and leaders in academic pediatrics.
Learn with us
Professional development activities provide valuable learning and CME opportunities to faculty, staff, and trainees as well as pediatricians throughout the region. We host weekly Pediatric Grand Rounds, annual conferences and lectures, and regular workshops.
Work with us
We are dedicated to recruiting new faculty and staff to help us improve the lives of children in Wisconsin and beyond. Internships and student employment opportunities in health care administration and research provide experiences for emerging professionals.
Featured Events and News
Recent News

Physician Scientist Training Pathway supports new residents who wish to combine medicine and scientific research
Every year when new students enter the UW School of Medicine and Public Health (SMPH), some combine their medical studies with scientific research, continuing often already extensive undergraduate research experience. Some choose to look for …
May 8, 2024
16 department members receive Research and Development Awards during fall/winter funding cycle
The Department of Pediatrics provided department members with a total of $167,611 during the fall/winter award cycle of its Research and Development (R&D) Awards. The purpose of the R&D Awards fund is to support research …
May 7, 2024
Reflecting its evolution and vision, the Division of Hospital Medicine adds ‘Complex Care’ to its name
Faculty members and fellows from the Division of Hospital Medicine and Complex Care. Front row, left to right: Sarah Webber, MD, Kirstin Nackers, MD; Mary Ehlenbach, MD; Laura Chen, MD; Heidi Kloster, MD; Ann Allen, …
May 7, 2024
Department of Pediatrics will host 18 Shapiro Summer Research Program students
This summer, 14 Department of Pediatrics faculty members from seven divisions will open their research groups to 18 medical students through the Shapiro Summer Research Program. Since 2002, the Shapiro Summer Research Program has provided …
May 6, 2024
Pediatrics faculty share expertise: April 2024
Department of Pediatrics faculty members are experts in child health, parenting, vaccinations, and other related topics. They are frequently sought out by local, state, and national journalists for their insights. Below is a list of …
May 3, 2024
Brian Williams receives career development award to address vaping among adolescents and young adults
Brian Williams receives career development award to address vaping among adolescents and young adults Brian Williams, MD, assistant professor in the Division of Hospital Medicine and Complex Care, has been awarded a K08 grant from …
April 29, 2024
Dan Sklansky named chair-elect of the APPD Program Directors Executive Committee
The nominations committee of the Association of Pediatric Program Directors (APPD) named Dan Sklansky, MD, associate professor, Division of Hospital Medicine and Complex Care, and director of the Pediatrics Residency Program, chair-elect of the APPD …
April 29, 2024
A New Investigator Program grant was awarded to Rebecca Richards to investigate engineered cells to more effectively treat acute myeloid leukemia
Rebecca Richards, MD, PhD, assistant professor, Division of Hematology, Oncology, and Bone Marrow Transplant, is the recipient of one of eight New Investigator Program grants from the Wisconsin Partnership Program (WPP). Her project is entitled, …
April 25, 2024- More News...