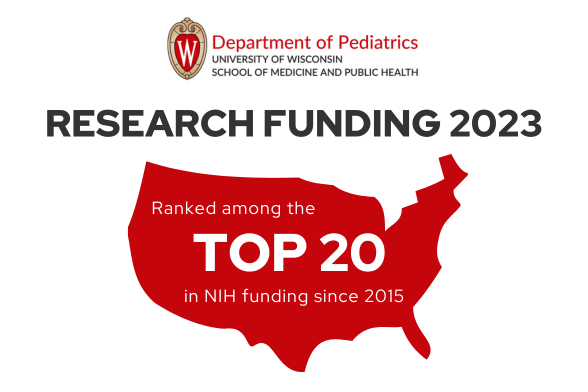
Welcome to the University of Wisconsin Department of Pediatrics
The Department of Pediatrics at the University of Wisconsin School of Medicine and Public Health promotes and enhances the health of children through outstanding clinical care, exemplary education of pediatric trainees, performance of cutting-edge research, and vigorous advocacy. It is the academic home to more than 200 faculty members across 16 subspeciality divisions.
Train with us
Formal training programs for medical students, residents, fellows, postgraduate researchers, and faculty provide subspecialty expertise to prepare the next generation of clinicians, scientists, scholars, and leaders in academic pediatrics.
Learn with us
Professional development activities provide valuable learning and CME opportunities to faculty, staff, and trainees as well as pediatricians throughout the region. We host weekly Pediatric Grand Rounds, annual conferences and lectures, and regular workshops.
Work with us
We are dedicated to recruiting new faculty and staff to help us improve the lives of children in Wisconsin and beyond. Internships and student employment opportunities in health care administration and research provide experiences for emerging professionals.
Featured Events and News
Recent News

A New Investigator Program grant was awarded to Rebecca Richards to investigate engineered cells to more effectively treat acute myeloid leukemia
Rebecca Richards, MD, PhD, assistant professor, Division of Hematology, Oncology, and Bone Marrow Transplant, is the recipient of one of eight New Investigator Program grants from the Wisconsin Partnership Program (WPP). Her project is entitled, …
April 25, 2024
Daniel Jackson inducted into the American Society for Clinical Investigation
Over the weekend of April 6, Daniel Jackson, MD, professor, Division of Allergy, Immunology, and Rheumatology, was inducted into the American Society for Clinical Investigation (ASCI). Founded in 1908, the ASCI is one of the …
April 24, 2024
WMAA honors David Bernhardt with Ralph Hawley Distinguished Community Service Award
The Wisconsin Medical Alumni Association (WMAA) honored its 2024 Distinguished Medical Alumni Award recipients during a banquet on April 19 at the Madison Concourse Hotel. Among the awardees was David Bernhardt, MD, professor, Division of …
April 24, 2024
After three decades of diverse service in genetics practice and regional outreach, David Wargowski will retire
From his arrival at the Department of Pediatrics in 1990 and for the next 30-plus years, David Wargowski, MD, professor in the Division of Genetics and Metabolism, has served tirelessly, addressing genetically based diseases and …
April 11, 2024
Wisconsin at PAS 2024
Faculty, trainees, and staff from the University of Wisconsin Department of Pediatrics will be presenting and contributing to 29 presentations and 35 posters at the 2024 Pediatric Academic Societies (PAS) meeting in Toronto, Canada, May …
April 9, 2024
Department of Pediatrics 2024 Research Week, including the Gerard B. Odell Lecture and Award, is coming in May
Next month, the Department of Pediatrics will offer its 2024 Research Week over five days, May 13–17. Through a combination of live-streamed lectures, in-person events, and interactive sessions with faculty members and trainees, audience participants …
April 8, 2024
Pediatrics faculty share expertise: March 2024
Department of Pediatrics faculty members are experts in child health, parenting, vaccinations, and other related topics. They are frequently sought out by local, state, and national journalists for their insights. Below is a list of …
April 8, 2024
James Padley and Philip Doll to take leadership positions in the SMPH Office of Informatics and Information Technology
Two members of the Department of Pediatrics computer services team have accepted leadership roles with the School of Medicine and Public Health Office of Informatics and Information Technology (SMPH IIT). James Padley, IT director I, has …
April 2, 2024- More News...