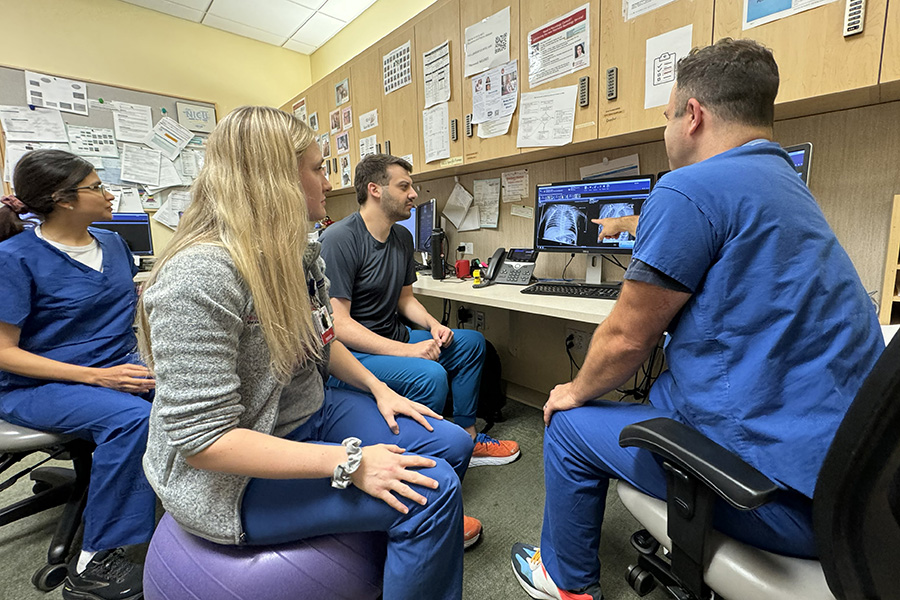
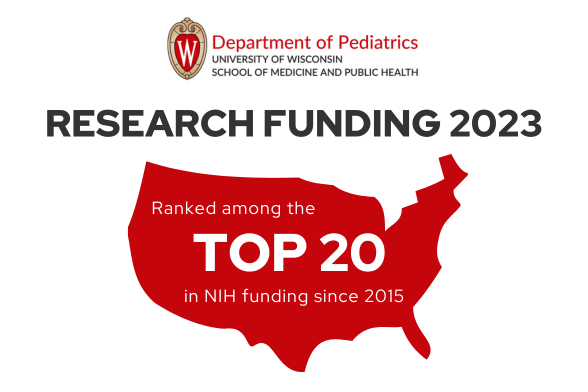
Welcome to the University of Wisconsin Department of Pediatrics
The Department of Pediatrics at the University of Wisconsin School of Medicine and Public Health promotes and enhances the health of children through outstanding clinical care, exemplary education of pediatric trainees, performance of cutting-edge research, and vigorous advocacy. It is the academic home to more than 200 faculty members across 16 subspeciality divisions.
Train with us
Formal training programs for medical students, residents, fellows, postgraduate researchers, and faculty provide subspecialty expertise to prepare the next generation of clinicians, scientists, scholars, and leaders in academic pediatrics.
Learn with us
Professional development activities provide valuable learning and CME opportunities to faculty, staff, and trainees as well as pediatricians throughout the region. We host weekly Pediatric Grand Rounds, annual conferences and lectures, and regular workshops.
Work with us
We are dedicated to recruiting new faculty and staff to help us improve the lives of children in Wisconsin and beyond. Internships and student employment opportunities in health care administration and research provide experiences for emerging professionals.
Featured Events and News
Recent News

After three decades of diverse service in genetics practice and regional outreach, David Wargowski will retire
From his arrival at the Department of Pediatrics in 1990 and for the next 30-plus years, David Wargowski, MD, professor in the Division of Genetics and Metabolism, has served tirelessly, addressing genetically based diseases and …
April 11, 2024
Wisconsin at PAS 2024
Faculty, trainees, and staff from the University of Wisconsin Department of Pediatrics will be presenting and contributing to 29 presentations and 35 posters at the 2024 Pediatric Academic Societies (PAS) meeting in Toronto, Canada, May …
April 9, 2024
Department of Pediatrics 2024 Research Week, including the Gerard B. Odell Lecture and Award, is coming in May
Next month, the Department of Pediatrics will offer its 2024 Research Week over five days, May 13–17. Through a combination of live-streamed lectures, in-person events, and interactive sessions with faculty members and trainees, audience participants …
April 8, 2024
Pediatrics faculty share expertise: March 2024
Department of Pediatrics faculty members are experts in child health, parenting, vaccinations, and other related topics. They are frequently sought out by local, state, and national journalists for their insights. Below is a list of …
April 8, 2024
James Padley and Philip Doll to take leadership positions in the SMPH Office of Informatics and Information Technology
Two members of the Department of Pediatrics computer services team have accepted leadership roles with the School of Medicine and Public Health Office of Informatics and Information Technology (SMPH IIT). James Padley, IT director I, has …
April 2, 2024
Tom Brazelton joins UW Health administration
Tom Brazelton, MD, MPH, professor, Division of Critical Care, and vice chair of quality, has recently accepted the position of UW Health Physician Administration Director for CDI, Coding & HIM. In this role, he will …
March 29, 2024
Three department members receive UW Health Safety Leadership Awards
During Patient Safety Awareness Week, March 11–15, the UW Health Patient Safety leadership team honored providers who show exemplary leadership and go above and beyond to improve patient safety in their work areas. Three Department …
March 22, 2024
Department of Pediatrics joins the national celebration for Resident Match Day
The University of Wisconsin Department of Pediatrics was excited to celebrate Match Day 2024 on March 15. Medical students and programs from across the country eagerly awaited match results with friends, families, and colleagues. Last …
March 15, 2024- More News...